Michael Bryant: More to the Disturbing Story of Alexis Lorenze — A Medical Nightmare
An alarming and profoundly troubling story in the medical community has recently sparked national attention and has trained yet another spotlight on the current state of affairs in hospital systems...
One-time or recurring donations can be made through Ko-Fi:
By Michael Bryant Global Research, September 26, 2024
An alarming and profoundly troubling story in the medical community has recently sparked national attention and has trained yet another spotlight on the current state of affairs in hospital systems across the United States.
This harrowing story concerns 23-year-old Alexis Lorenze and her experience after being admitted to University of California, Irvine Medical Center (UCIMC) in Orange, California, on September 10 for treatment of what was diagnosed as paroxysmal nocturnal hemoglobinuria (PNH).
What is PNH? Respected writer Marcella Piper-Terry, an expert on vaccines and vaccine injuries, simplified and summarized the Cleveland Clinic’s explanation of PNH in one of her recent Substack posts:
Paroxysmal noctural hemoglobinuria (PNH) is a rare blood disorder that happens when part of your immune system attacks and damages red blood cells and platelets. Fewer than 20 years ago, PNH was a debilitating disease treated with blood transfusions. Even so, PNH put people at risk for serious and sometimes life-threatening illnesses. Most people lived 10 to 22 years after their diagnosis. But today, people with PNH receive innovative treatment that protects their blood cells and reduces their risk of serious illness. People with PNH can expect to live as long as someone who doesn’t have the disease.
In a Facebook video that Alexis Lorenze made and posted on September 15 and that Piper-Terry embedded in her aforementioned Substack article, Lorenze said she was diagnosed with PNH last January. She also said the blood disorder “was triggered by a cough syrup she was prescribed,” to quote Piper-Terry.
While there is no solid evidence corroborating or denying that a prescription cough medicine caused this rare disease, that explanation seems rather implausible. This raises the question as to how PNH is diagnosed in the first place.
PNH is generally diagnosed by examining blood cells to see if they lack the surface proteins that are characteristically absent in people with the disease. The examination is done in a laboratory test called flow cytometry.
The disease is primarily treated with medications that “block the activation of the complement system” in order to ease symptoms and reduce the risk of life-threatening complications.
There are currently six complement-blocking medications approved to treat PNH in the US. The Cleveland Clinic website lists the six:
Soliris (eculizumab)
Ultomiris (ravulizumab-cwvz)
PiaSky (crovalimab-akkz)
Empaveli (pegcetacoplan)
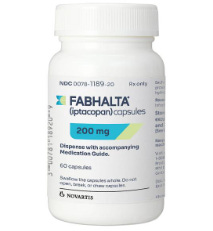
Fabhalta (iptacopan)
Voydeya (danicopan)
In her video, Lorenze said the hospital required that she take the meningitis vaccine in order to receive treatment for the PNH. She also alleged that doctors then ordered that she not only be given the vaccine for meningitis but also vaccinated against pneumonia and tetanus. She said the doctors coerced her into taking all three shots simultaneously.
As there is currently no single tetanus shot, this means that if in fact she was given a shot for “tetanus,” the inoculation she most likely received was the three-in-one Tdap vaccine or the Td vaccine.
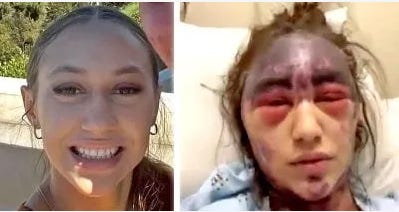
Speaking on camera, Lorenz and her family verified that within minutes of receiving the vaccines, she experienced severe adverse reactions. She lost vision in both her eyes. She experienced internal bleeding and numbness. She coughed up blood and shook uncontrollably. Meanwhile, painful dark purple patches quickly developed and spread across much of her body. Her family claimed that the entire time her condition was rapidly deteriorating, hospital staff delayed treatment for her adverse reactions.
In a more recent report—which differs from what Alexis and her sister said they were told— registered nurse Angela Wulbrecht, who was brought in to advocate for the patient, said she was informed that “Alexis received the Meningococcal ACWY vaccine (This type of vaccine is a quadrivalent, meningococcal vaccine) the Meningococcal B vaccine, along with the Haemophilus B vaccine.”
News reports on her case have, understandably, focused on the possibility that Lorenze experienced vaccine damage. After all, there were already documented medical concerns about prescribing the meningitis vaccine to PNH patients.
Whichever exact vaccines were given, it should be noted that vaccines are not tested for how safe it is to co-administer them nor are they tested for how they interact when integrated with other drugs. Without a full assessment for combined or cumulative toxicities and possible reactogenicity, a comprehensive medical evaluation is unattainable.
However, it appears there may be more to this tragic story than simply an adverse—albeit massive—reaction to a series of vaccines.
Indeed, the most concerning question being asked is: Could other experimental drugs or treatments have been given to Lorenze? And, if so, could these drugs or treatments have caused or exacerbated her condition?
Of particular note is the fact that UCIMC attending physician Dr. Zahra Pakbaz is currently involved in a clinical trial for a new drug being promoted as “the first single oral therapy available for PNH.”
That new drug is Novartis’ FABHALTA® (iptacopan). It received FDA fast track approval on August 8—roughly a month before Lorenze was admitted to the hospital.
The risk profile for FABHALTA is suspect, though. The product’s own website warns, “It is not known if FABHALTA is safe and effective in children with PNH.”
Another notable feature of FABHALTA is that vaccinations against Streptococcus pneumoniae and Neisseria meningitidis are “required at least 2 weeks before the first dose.” But, the website goes on to say, if you have not completed your vaccinations and FABHALTA must be started right away, “you should receive the required vaccinations as soon as possible.”
FABHALTA is no ordinary daily medication. Rather, it is the first of Novartis’ trio of IgA nephropathy (IgAN) drugs. Novartis projects FABHALTA is on course to be a blockbuster product.
Analysts at the investment banking firm Jefferies seem to agree. They predicted that FABHALTA could hit $3.6 billion in peak annual sales “[i]f it gets approved for all its target indications, which along with PNH and IgAN include atypical haemolytic uraemic syndrome (aHUS), C3 glomerulopathy (C3G), and idiopathic membranous nephropathy (IMN).”
Not only is FABHALTA expected to play a big role in Novartis’ financial portfolio, but it turns out that UCIMC’s Dr. Pakbaz plays a significant role in the drug’s marketability.
“Over a two-year span,” reports an article on Oncology Tube, “Pakbaz’s clinic, which served solely benign hematology patients, registered a significant demand that crossed various zip codes and cities.”
The same article reported on a presentation Dr. Pakbaz gave at the March 2024 annual meeting of the American Society of Hematology (ASH). She described the advancements being made in benign hematology, highlighted the role that Non-Malignant Hematology Clinics play in enhancing patient outcomes, and spoke of “the remarkable potential of gene therapies to heal diseases that were once deemed incurable.”
During her presentation, Dr. Pakbaz spent significant time touting the benefits of FABHALTA® (iptacopan) praising its performance compared to other similar drugs.
Perhaps her promotion of Novartis’ prized cash cow merits further scrutiny. Not so long ago—July 2020, to be exact—Novartis paid out $678 million to settle a fraud lawsuit for operating sham speaker programs. It turns out the drug company had paid over $100 million to doctors to unlawfully induce them to prescribe Novartis drugs.
A recent article in The Defender cited a study published on September 1 in JAMA Internal Medicine which showed that “among 5,533 U.S. cardiology fellows, 73% received ‘industry marketing payments’ in the year before graduating and 88% received payments in the first few years after they graduated.”
Unfortunately, such conflicts of interest and questionable ethical behavior in the medical, scientific, and regulatory communities are all too common and have been widely documented.
In light of the many conflicts of interest that reside in doctors’ offices, university medical systems, regulatory bodies, and pharmaceutical companies, a few tough questions must be asked in the case of Dr. Pakbaz and the Novartis drug her clinic is testing:
(1) Is it possible Dr. Pakbaz was looking to shape diagnoses in order to create more subjects who would fit the objectives of the drug trials?
(2) Is it possible that Alexis Lorenze was caught in a dragnet search for patients to participate in this trial?
(3) Is it possible Lorenze was unwittingly involved in a clinical trial without her consent?
In an interview with Polly Tommey at CHD-TV, Lorenze’s sister, Samantha Lorenze, told Tommey that a nurse at the UCI hospital mentioned that Alexis had been given a different medication outside of the vaccine vials. The sister also alleged that hospital staff called Lorenze “a science experiment.”
In the online publication Intelligencer, Alexis Lorenze was quoted as saying, “When I learned of the cost of the treatment, Iptacopan ($47,000.00 per month), I expressed concern to the hospital staff that I couldn’t afford it, and then they pushed me to go home, even though my health was unstable.”
If this is true, the problem may well extend beyond the possibility that poor decisions are being made by members of the medical community. Indeed, it appears the problem is more deeply rooted, widespread, and systemic. Indeed, Lorenze’s advocate, Angela Wulbrecht, RN, characterizes the medical system as an enterprise that “caters to the perverse relationship doctors have with vaccine manufacturers that result in financial conflicts of interest at the expense of patients like Alexis.”
At present, there appear to be more questions than answers. What is immediately and desperately needed, in our opinion, is a fully independent investigation into potential hospital/doctor misconduct, including possible conflicts of interest.
Full disclosure must be made to the Lorenze family. That disclosure must include the timeline and the procedures involved in Alexis Lorenze’s diagnosis as well as all the medications and treatments she was given.
This article was originally published on Health Freedom Defense Fund.
Michael Bryant is a freelance journalist/activist and researcher who presently focuses primarily on issues surrounding health freedom. His work has appeared on HealthFreedomDefense.org. He is a frequent contributor to Global Research.
The original source of this article is Global Research
Copyright © Michael Bryant, Global Research, 2024
Related articles:












PNH is on the nine pages of Adverse Events for the COVID shots. Ask if she was COVID vaccinated. The Adverse Events start on page 30 of the following Pfizer document both the CDC and Pfizer were trying to hide for 75 years: https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf
I have serious doubts about "full disclosure" regarding ANY hospital treatment in the post-Covid era. I would have to be on death's doorstep to willingly submit myself to the "care" we have long relied on in a hospital environment. The risk is heavily weighted against the benefits (i.e: paxlovid, remdesivir), ventilators, etc. Scary times to be living in, when it comes to our health.