DISTURBING: FDA Clears Investigational New Drug Application for Inhaled COVID Biologic
Inhalation COVID Vaccine Trials Funded With $5 Billion from NIH
One-time or recurring donations can be made through Ko-Fi:
By Natasha Hobley February 10, 2025
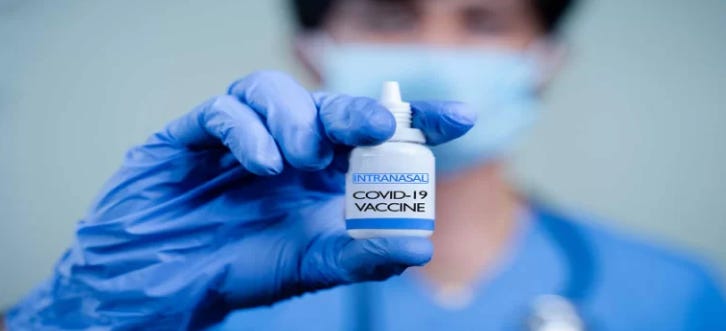
Biotech company Ocugen, Inc. has announced that the U.S. Food and Drug Administration (FDA) has cleared their Investigational New Drug (IND) application for a novel inhaled vaccine against SARS-CoV-2.
The biologic uses novel chimpanzee adenovirus-vectored (ChAd36) technology. The approval will allow the company to initiate Phase I clinical trials for the inhaled vaccine, OCU500. The Phase I trial is expected to begin in the second quarter of this year and will be sponsored and conducted by the National Institute of Allergy and Infectious Diseases (NIAID).1
The Phase I trial will involve 80 adults ages 18-64 and will test a dual delivery approach via inhalation and intranasal routes, targeting the natural infection route of respiratory viruses. Of the 80 participants, 40 will be assigned to a low-dose group and the remaining 40 will be assigned to a high-dose group. Within each group, 20 subjects will receive the vaccine via inhalation, and the other 20 subjects will receive the intranasal form. According to the company’s press release, the primary aim of the study is to determine safety, while the secondary and exploratory endpoints include antibody production and the number of breakthrough COVID-19 infections.1
The Ocugen press release states that the ChAd36 vector demonstrates superior dose efficiency that will require one-fifth of the dose compared to traditional intramuscular delivery. The company hopes for a potential to expand to other respiratory diseases such as influenza, RSV, and bird flu.1
Inhalation COVID Vaccine Trials Funded With $5 Billion from NIH
The collaboration between the biotech company Ocugen and the NIAID, which falls under the U.S. National Institutes of Health (NIH), is part of the agency’s NextGen project. This $5 billion initiative, which was announced in April 2023, is a collaboration between the government and biotech companies aiming to help “address gaps in the nation’s COVID biologic and therapeutic capabilities.”1 Project NextGen includes three strategic areas: strengthen, treat, and enable.2
The strategy to “strengthen” includes advancing next generation COVID biologics with more breadth, protection, and capabilities. “Treat” involves having better durability against new variants and treatments for those who do not respond to vaccination. Falling under the strategy goal to “enable” includes advancing better and innovative vaccine approaches and faster vaccine manufacturing.2
Taxpayer Funded NextGen to “Revitalize” COVID-19 Prevention Program
An article published in the journal Clinical Infection Diseases outlining the NextGen initiative states:
The bedrock of this program is the U.S. government’s goal to revitalize the pipeline of solutions to address the most pressing gaps in COVID prevention and treatment capabilities for the nation”2
NextGen funding for Ocugen will use tax-payer dollars to cover the full cost of the Phase 1 inhalation COVID vaccine clinical trial, including operations and related analysis.1
The Ocugen press release maintains that COVID disease remains a public health threat and a significant burden to the United States. The Ocugen press release for the new inhaled vaccine states that the U.S. Centers for Disease Control and Prevention (CDC) estimated that there were 14,000 to 25,000 COVID infections deaths from October 2024 to Jan. 11, 2025. For comparison, there were 28,000 influenza related deaths during the roughly six-month flu season in 2024,3 and in 2024, there were 931,578 cardiovascular-related deaths.4
Mike Shine, senior Commercial Vice President at Ocugen, stated:
COVID-19 remains a real public health concern, and an increasing number of studies are showing the benefit of mucosal vaccines that attack the virus where it enters the body—through the nose and mouth—to give better and longer protection. We look forward to this important next step in potentially providing a more durable and safer option to help prevent infection and transmission of COVID-19 regarding various variants of concern.1
Shine has held leadership positions with Novapharm Therapeutics, Colgate Oral Pharmaceutical, and Pfizer with over 35 years of experience. He led the “successful commercial launch of the global $6 billion Prevnar vaccine franchise while with Pfizer Vaccines,” and has “driving sales in excess of $2 billion” according to an Ocugen press document released via GlobeNewswire when Shine was hired by the company.5
References:
1 Hamilton T. Ocugen, Inc. announces investigational new drug application in effect after review by FDA to initiate phase 1 clinical trial evaluating first-in-class OC500 inhaled vaccine candidate for COVID-19. Yahoo Finance Jan.27, 2025.
2 Hofmeyer K. Project NextGen: Developing the next generation of COVID-19 vaccines and therapeutics to respond to the present and prepare for the future. Clinical Infection Diseases Feb. 13, 2024.
3 Correia S. Ocugen, Inc. announces Michael Shine as senior vice president, commercial. Global Newswire June 10, 2021.
4 Press Release. Ocugen, Inc. Announces Investigational New Drug Application in Effect After Review by FDA to Initiate Phase 1 Clinical Trial Evaluating First-in-Class OCU500 Inhaled Vaccine Candidate for COVID-19. Ocugen Jan. 27, 2025.
5 GlobeNewswire. Ocugen Inc. Announces Michael Shine as Senior Vice President, Commercial. Ocugen June 10, 2021.
Source: thevaccinereaction.org
Related articles:









Those monsters will not stop until they are physically stopped by turning off the money supply and being removed from their positions. They are corrupt gangsters working for the corrupt gangsters of pharma. Trials and the death penalty for anyone convicted. They murdered with reckless abandon in 2021 and need to be stopped.
Give them what they give us…death penalty! 👊🏻