
Moderna SEC Filing: We May Be Delayed or Prevented From Receiving Full Regulatory approval. Unexpected Safety Issues Could Significantly Damage Our Reputation and That of Our mRNA Platform
"We haven't received approval to market any investigational medicine, other than our COVID19 vaccine, our current or future development candidates may never obtain regulatory approval"
On February 25, 2022, Moderna released its Annual SEC Filing.
Just like Pfizer in its SEC FIling, Moderna admits that due to safety and efficacy concerns their investigational mRNA Covid - 19 Gene Therapy “Vaccines” may be delayed or prevented from receiving full regulatory approval.
Excerpts:
Page 59
Item 1A. Risk Factors
We may be delayed or prevented from receiving full regulatory approval of our COVID-19 vaccine in certain jurisdictions or for certain demographics
Efficacy, effectiveness, safety, and immunogenicity data with respect to our COVID-19 vaccine, as well as real-world evidence, continue to accumulate. Further results from clinical trials, as well as the experience of vaccinated individuals, could show diminished protection compared to the results released to date, as efficacy and antibody persistence wane over time.
Additionally, we may observe new, more frequent or adverse events of greater severity in subjects participating in ongoing clinical trials or among those individuals vaccinated with our COVID-19 vaccine. For example, some studies have suggested that our vaccine may be associated with higher rates of myocarditis and pericarditis in young males compared to other COVID-19 vaccines.
Unexpected safety issues could significantly damage our reputation and that of our mRNA platform, and lead to other issues, including delays in our other programs, the need to re-design our clinical trials and the need for significant additional financial resources.
The assays used to estimate the effectiveness of COVID-19 vaccines have only recently been developed and continue to evolve. Validation reports for these assays have been submitted for review to regulatory agencies.
Results obtained in clinical studies of mRNA-1273 with later versions of these assays may be less positive than the results we have obtained to date.
The future results in clinical studies of mRNA-1273 may not be as positive when compared to the antibody levels in other blood samples.
We may be unsuccessful in developing future versions of our COVID-19 vaccine to protect against variants of the SARS-CoV-2 virus, or booster doses of our vaccine may not protect against such variants, and a market for vaccines and boosters against these variants may not develop.
…Additionally, administration of booster doses of our vaccine may prove to be ineffective, or less effective than desired, against certain variants. We have several development candidates against variants of concern, and may develop others in the future. If these efforts are unsuccessful, we are slower to develop variant-specific vaccines than competitors, or these vaccine candidates prove less effective than competitors’ vaccines, these shortcomings may lead to reputational harm, loss of market share, and adverse financial results. (Safe and Effective!)
Page 64
mRNA drug development has substantial clinical development and regulatory risks due to the novel nature of this new class of medicines, and the negative perception of the efficacy, safety, or tolerability profile of any investigational medicines that we or others develop could adversely affect our ability to conduct our business, advance our investigational medicines, or obtain regulatory approvals.
No mRNA medicine has been granted full or conditional approval by the FDA or other regulators, other than COVID-19 vaccines. Successful discovery and development of mRNA medicines by us or our strategic collaborators is highly uncertain and depends on numerous factors, many of which are beyond our or their control. We constantly make business decisions and take calculated risks to advance our development efforts and pipeline, including those related to mRNA technology, delivery technology, and manufacturing processes, which ultimately may be unsuccessful. (Safe and Effective!)
Page 65
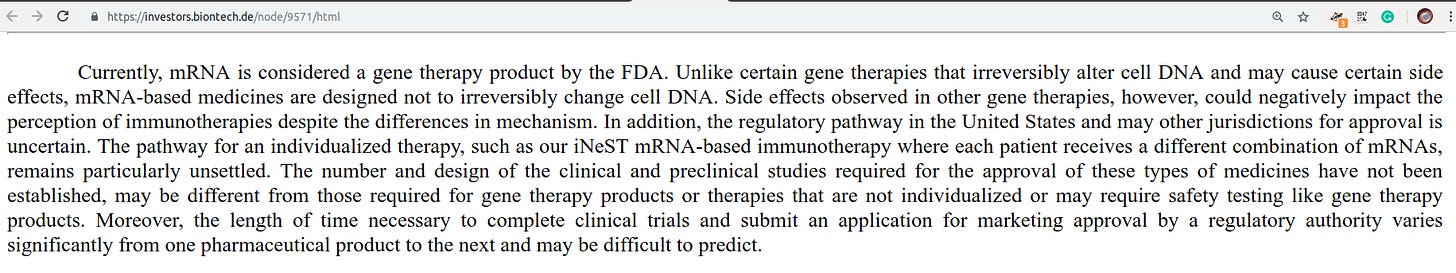
Some of our investigational medicines are classified as gene therapies by the FDA and the EMA. The association of our medicines with gene therapies could result in increased regulatory burdens, impair the reputation of our investigational medicines, or negatively impact our platform or our business.
There have been few approved gene therapy products in the United States or foreign jurisdictions, and there have been well-reported significant adverse events associated with their testing and use. Regulatory requirements governing gene and cell therapy products have evolved and may continue to change in the future, and the implications for mRNA-based therapies are unknown (Safe and Effective!)
From BioNTech SEC Filing:
Bayer executive: mRNA shots are ‘gene therapy’ marketed as ‘vaccines’ to gain public trust
You can download the entire document here: Moderna Annual Report SEC Filing
In this twisted and evil world a company that never developed a product prior to the plandemic, can play God by calling its mRNA technology “The software of life”, present a strategy to bring to market mRNA based pan-respiratory annual booster vaccine, vaccines against latent viruses, seasonal influenza vaccine (mRNA-1010, mRNA-1011, mRNA-1012, mRNA-1020 and mRNA-1030), cancer vaccine, and therapeutics based on mRNA-encoded proteins while admitting that implications for mRNA-based therapies are unknown.
Now they are coming for our babies:
Implications like injuries and deaths don’t matter when you have strategic alliances with government organizations and foundations such as Defense Advanced Research Projects Agency (DARPA), Biomedical Advanced Research and Development Authority (BARDA), and The Bill & Melinda Gates Foundation"
Page 41
Page 42
Bill Gates’ Foundation Working with DARPA on Gene Editing
In an interview with James Corbett of the Corbett Report, Johnathan Latham discusses The Bill & Melinda Gates Foundation’s disturbing ties to the United States government. DARPA (Defense Advanced Research Projects Agency) an agency of the Department of Defense is working closely with Gates’ Foundation to introduce genetic modifications into various species. Continue reading
Related articles:
Dr. Michael Yeadon: THIS MUST STOP! Pfizer Documents Show FDA Knew of Death Risk
Premeditated GENOCIDE: Pfizer mRNA Integrates into your DNA
Pfizer Offers Millions in Bribes To Buy the Silence of Outspoken Doctors
Your support is greatly appreciated!
If you wish to support by contributing a different amount from the substack yearly subscription, you can Donate via Ko-fi, where you can buy as many “coffees” as you like, monthly or one-time donations are accepted.




















At the same time they are asking the FDA for EUA for kids under five, they are so disgusting!
Thanks. I appreciate that you present the information succinctly and provide links. I appreciate your work.