BioNTech 2022 SEC Filing: We May Not Be Able To Demonstrate Sufficient Efficacy or Safety of Our COVID-19 Vaccine. Significant Adverse Events Could Delay or Prevent Regulatory Approval
A notable change: " Undesirable Side Effects" in 2021 Filing was upgraded to "Significant Adverse Events"
While we are eagerly waiting for the next Pfizer document dump, BioNTech released its annual SEC Filing.
Just like in the 2021 Annual SEC Filing, Pfizer admits that due to safety concerns and the inability to demonstrate sufficient efficacy, they are not likely to receive regulatory approval.
A notable change: " Undesirable Side Effects" in 2021 Filing was upgraded to "Significant Adverse Events".
Will this damning documented admission be ignored again by the MSM and the majority of so-called Alternative media the same way they ignored BioNTech’s 2021 SEC Filing?
Get ready for the never-ending state of emergency that will allow criminal world governments to mandate experimental Gene Therapy poison under the Emergency Use Authorisation for years to come.
Excerpts:
Page 6
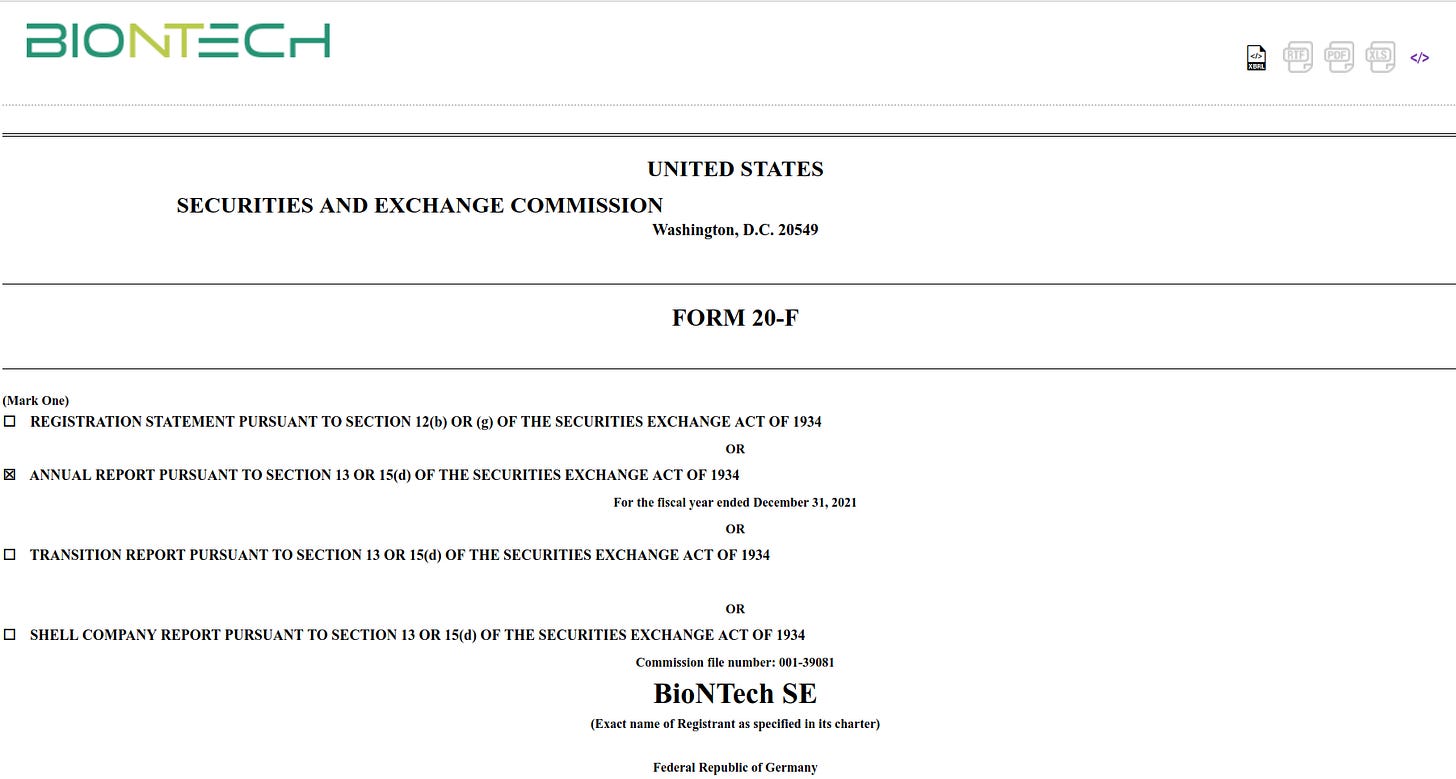
Risk Factors
Our revenue depends heavily on sales of our COVID-19 vaccine, and our future revenues from our COVID-19 vaccine are uncertain.
We may not be able to demonstrate sufficient efficacy or safety of our COVID-19 vaccine and/or variant-specific formulations to obtain permanent regulatory approval in the United States, the United Kingdom, the European Union, or other countries where it has been authorized for emergency use or granted conditional marketing approval.
Significant adverse events may occur during our clinical trials or even after receiving regulatory approval, which could delay or terminate clinical trials, delay or prevent regulatory approval or market acceptance of any of our product candidates.
You can download the entire document here: BioNTech SEC Filing
BioNTech 2021 SEC Filing:
mRNA "Vaccines" Are Gene Therapy. May cause Undesirable Side Effects That Could Delay Or Prevent Their Regulatory Approval According To BioNTech SEC Filing
The Truth About "Safe and Effective" mRNA "Vaccines" Hidden In Plain Sight
Related articles:
Pfizer Offers Millions in Bribes To Buy the Silence of Outspoken Doctors
Dr. Michael Yeadon: THIS MUST STOP! Pfizer Documents Show FDA Knew of Death Risk
Premeditated GENOCIDE: Pfizer mRNA Integrates into your DNA
Your support is greatly appreciated!
If you wish to support by contributing a different amount from the substack yearly subscription, you can Donate via Ko-fi, where you can buy as many “coffees” as you like, monthly or one-time donations are accepted.





While this is obviously theater of the absurd, is it possible that this language is standard in drug SEC filings so they cover their bases?
Wow if this admission doesn’t convince the covidians I don’t know what will 😷